Overview
Metastasis is the leading cause of death in cancer patients. The formation of metastasis, or even secondary tumors, is remarkably influenced by different types of tumor-stroma interactions, in which stromal components of the tumor microenvironment can influence the behavior of cancer cells. Hormone-dependent breast cancers frequently become refractory to initially effective hormonal treatments, thus eventually requiring chemotherapy. Chemotherapy, in fact, still represents the most common option for advanced breast cancers. However, it has been demonstrated that chemotherapy itself, despite triggering shrinkage of primary tumors, can enhance metastasis through the induction of epithelial-mesenchymal transition (EMT). Moreover, cancer cells can develop drug resistance, resulting in treatment failure and recurrence.
The main interest of our group is to explore the molecular mechanisms underlying chemoresistance and critical for the metastatic process, especially in breast cancer. A particular attention is paid on the gene expression network reprograming driven by master regulator transcription factors with a prominent role in tumor biology, such as p53, ETV7, and NF-κB.
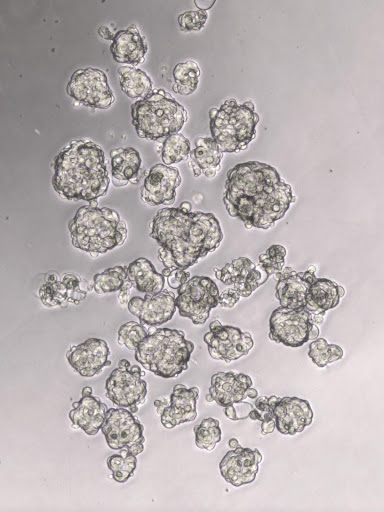
Moreover, the laboratory interests are also based on the study of Cancer Stem Cells (CSCs). CSCs represent a cellular population part of the tumor cell mass with self-renewal properties linked to cancer aggressiveness. These cells are considered to be intrinsically highly chemoresistant, and CSCs have been proposed to play a pivotal role in cancer recurrence after chemotherapy. However, although studied for several years, the mechanisms underlying CSCs are not completely appreciated yet, and their specific targeting is still complicated. Moreover, even tumor dormancy has been associated with CSCs. Therefore, uncovering the pathways and mechanisms involved in CSCs enrichment or drug resistance or unwanted side effects associated with chemotherapy is an urgent and critical aim for cancer research oriented to improve treatment efficacy.
The activities of the Laboratory of Molecular Cancer Genetics are primarily focused on the development of genome-wide, 3D, and transcription-based assays using cancer-derived cell lines and primary cells. Furthermore, we carry out different molecular analyses on biopsies derived from cancer patients.
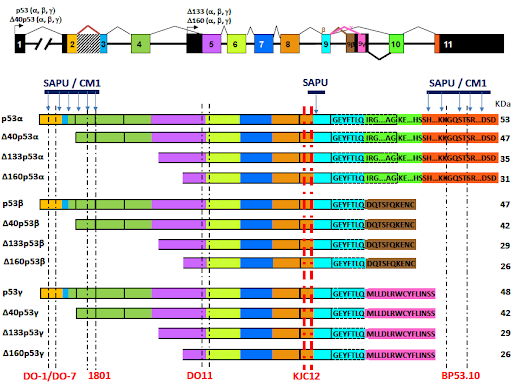
Research directions
The main research directions are focused on the analysis of gene expression in breast as well as colon cancer patients and cell lines, in vitro 2D and 3D studies and models of metastasis and angiogenesis.
-
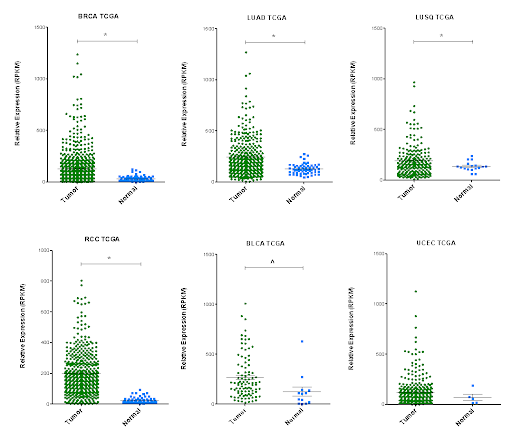
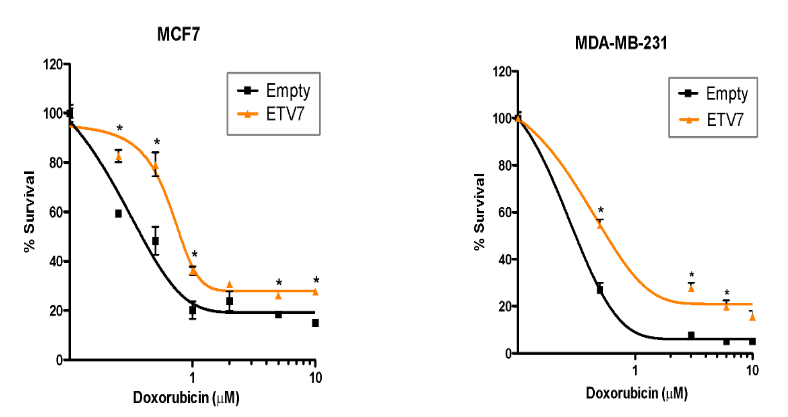
Identification of the molecular mechanisms underlying the increased chemoresistance and stemness mediated by the transcriptional network ruled by the transcriptional repressor ETV7.
-
Determination of the p53 isoforms’ expression, subcellular localization, and functions in cancer cells.
-
Definition of the prognostic value of identified gene signatures in breast and colon cancer patients.
-
Role of EMT as mechanism of resistance to chemotherapy.
-
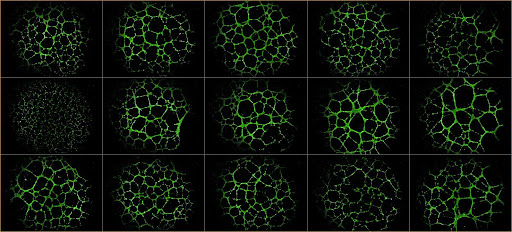
Defining the role of microenvironment in tumor angiogenesis and metastasis formation.
Group members
- Yari Ciribilli, PI
- Erna Marjia Meškytė, PhD student
- Yassine Khalij, PhD student
- Sabiha Keskas, visting PhD student
Motivated candidates, including undergraduate, master and doctoral students interested in the research topics, are encouraged to contact the PI (yari.ciribilli [at] unitn.it).
Collaborations
- Juergen Borlak, Hannover Medical School (MHH), Germany
- Daniel Menendez, National Institute for Environmental Health Sciences (NIEHS, NIH), Research Triangle Park, NC, USA
- Jean-Christophe Bourdon, Dundee Cancer Centre, University of Dundee, Scotland
- Ira-Ida Skvortsova (Medical University of Innsbruck, Austria)
- Eva Martinez-Balibrea (Catalan Institute of Oncology, Badalona, Spain)
- Neda Slade (Ruder Boskovic Institute, Zagreb, Croatia)
- Stefano Indraccolo (Istituto Oncologico Veneto, Padoa, Italy)
- Mattia Barbareschi, Santa Chiara Hospital, Trento, Italy
- Antonella Ferro, Santa Chiara Hospital, Trento, Italy
- Gilberto Fronza, National Institute for Cancer research (IST), Genoa, Italy
Funding
Bando: PRIN 2022 (D.D. 104/22)
New therapeutic strategy for the treatment of ovarian, pancreatic and pleural cancers
Yari Ciribilli, Responsabile di Unità
Codice Protocollo: 2022ZTM4NB CUP: E53D23012910001

Selected publications
Tadijan A, Precazzini F, Hanžić N, Radić M, Gavioli N, Vlašić I, Ozretić P, Pinto L, Škreblin L, Barban G, Slade N, Ciribilli Y. Cancers (Basel). 2021 Oct 18;13(20):5231. doi: 10.3390/cancers13205231. PMID: 34680379
Pezzè L, Forcato M., Pontalti S., Badowska K.A., Rizzotto D., Skvortsova I., Bicciato S., Ciribilli Y. ETV7 regulates breast cancer stem-like cell plasticity by repressing IFN-response genes. Cell Death and Disease (2021) 12:742. Altered Expression of Shorter p53 Family Isoforms Can Impact Melanoma Aggressiveness.
Meškytė EM, Keskas S, Ciribilli Y. MYC as a Multifaceted Regulator of Tumor Microenvironment Leading to Metastasis. Int J Mol Sci. 2020 Oct 18;21(20):7710. doi: 10.3390/ijms21207710.
Ozretić P, Hanžić N, Proust B, Sabol M, Trnski D, Radić M, Musani V, Ciribilli Y, Milas I, Puljiz Z, Bosnar MH, Levanat S, Slade N. Expression profiles of p53/p73, NME and GLI families in metastatic melanoma tissue and cell lines. Sci Rep. 2019 Aug 28;9(1):12470.
Alessandrini F*, Pezzè L*, Menendez D, Resnick MA, Ciribilli Y. ETV7-Mediated DNAJC15 Repression Leads to Doxorubicin Resistance in Breast Cancer Cells. Neoplasia. 2018 Aug;20(8):857-870.
Pinazza M.*, Ghisi M.*, Minuzzo S., Agnusdei V., Fossati G., Ciminale V., Pezzè L., Ciribilli Y., Pilotto G., Venturoli C., Amadori A. and Indraccolo S. Histone deacetylase 6 controls Notch3 trafficking and degradation in T-cell Acute Lymphoblastyc Leukemia cells. Oncogene. 2018. Jul;37(28):3839-3851.
Ciribilli Y., Borlak J. Oncogenomics of c-Myc transgenic mice reveal novel regulators of extracellular signaling, angiogenesis and invasion with clinical significance for human lung adenocarcinoma. Oncotarget. 2017 Oct 23;8(60):101808-101831.
Bisio A., Zámborszky J., Zaccara S., Lion M., Tebaldi T., Sharma V., Raimondi I., Alessandrini F., Ciribilli Y.*, Inga A.*. Cooperative interactions between p53 and NFkB enhance cell plasticity. Oncotarget. 2014 Oct 21.
* = equal contribution
