Overview
The development and the proper functionality of the central nervous system rely on a complex molecular and cellular machinery. Even minor alteration might have crucial impact on physiology and bring to disorders. We specially focus on Parkinson’s disease (PD) and psychiatric disorders such as major depression (MDD). PD is the second most common neurodegenerative disorder worldwide. To date, the most effective therapeutic approach to PD is based on the administration of L-DOPA which alleviate motor symptoms, but do not halt disease progression. Major depressive disorder (MDD) affects approximately 40.3 million people across Europe commonly manifesting in young adult age, and it is more frequent in women. Recent evidence indicates that MDD is caused by aberrant functional connection among various brain regions. There is urgent need to better understand the molecular mechanisms that lead to PD and MDD to design effective disease-modifying therapies. Our research aims at investigating the molecular bases of these diseases.
Research directions
We use a multidisciplinary approach, ranging from in vitro functional assays to genetic animal models, to study the molecular bases of neurological disorders:
-
Modelling PD
Mutations in Lrrk2 and Parkin genes are among most common genetic cause of PD. LRRK2 encompasses several functional domains, including a kinase domain. Parkin is an E3 ubiquitin-protein ligase involved in mitochondrial homeostasis, controlling both degradation of dysfunctional mitochondria and mitochondrial biogenesis. We leverage on two complementary mice models validate in our labs, the BAC hLRRK2 G2019S and Parkin R274W lines. We reported that mice overexpressing human LRRK2 G2019S (hG2019S) exhibit an age dependent motor dysfunction and neuronal death in the substantia nigra pars compacta (Pischedda et al. 2021). Parkin R274W mice show an age-related PD phenotype, including motor impairment, TH loss, astrogliosis, and synuclein deposition. We are exploring the role of the autophagy lysosomal system and of the glympathic system within disease progression.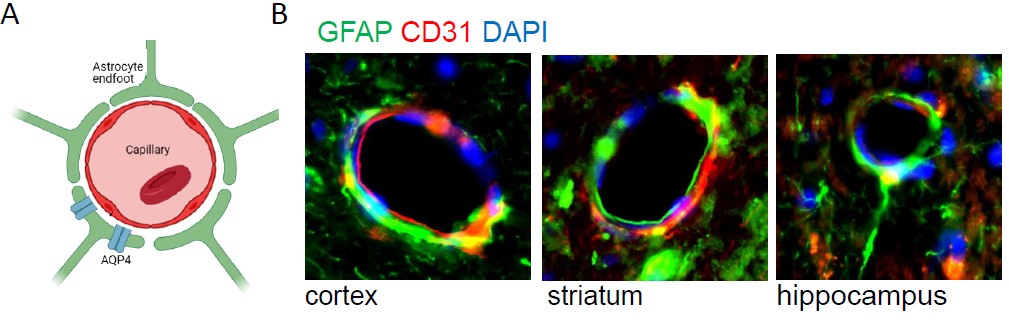 .
. -
Connectivity defects in psychiatric disorders
Genetic factors play important roles in the development of MDD. The Neuronal growth regulator 1 (NEGR1) gene has emerged as one of the most important genetic cause of MDD. In particular, there is a clear link between MDD and elevated levels of NEGR1. NEGR1 codes for a protein that is expressed on the surface of neurons, the major cells in the brain, where it dictates the connection with other cells. Our studies indicate that NEGR1 controls neuron migration and maturation via the FGFR2 receptor and downstream ERK and AKT pathways. We have generated a unique biobank of human iPSC carrying deletions in Negr1 locus. We plan to characterize the pathway modulated by Negr1 in complementary disease relevant models, including patients derived induced neurons and astrocytes.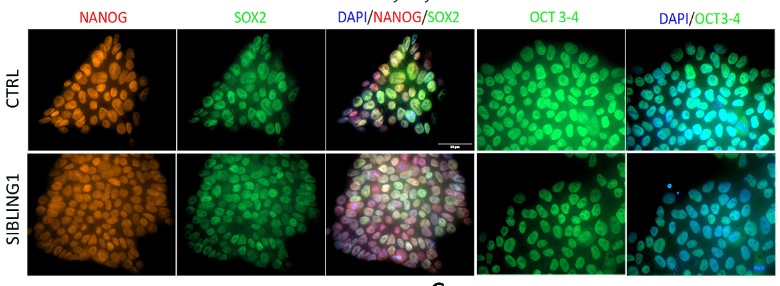
Group members
- Giovanni Piccoli, PI
- Vasvi Tripathi, post-doc
- Adriano Lama, post-doc
- Sandra Abdullatef, post-doc
- Alessia Ghirelli, pre-doc
The Laboratory of Biology of Synapses has been established in 2015 through an agreement between CIBIO and the Fondazione Telethon.
Collaborations
- Elisa Greggio, Università di Padova, Padova, Italia
- Laura Cancedda, IIT , Genova, Italia
- Mattia Volta, EURAC, Bolzano, Italia
- Bruno Giometto, APSS Trento, Italia
- Johannes Gloeckner, DZNE, Tubingen, Germany
- Franco Onofri, Università di Genova, Genova, Italia
- Marcus Reindl, University of Innsbruck, Innsbruck, Austria
Funding
Bando: PRIN 2022 (D.D. 104/22)
Dysfunctional autophagy-lysosomal pathways in Parkinson's disease: insights from Leucine-rich repeat kinase 2 (LRRK2)
Giovanni Piccoli, Responsabile di Unità
Codice Protocollo: 2022LRHCW CUP: E53D23011140006

Selected publications
Szczurkowska,J., Pischedda,F., Pinto,B., Managò,F., Haas,C.A., Summa,M., Bertorelli,R., Papaleo,F., Schäfer,M.K., Piccoli,G., Cancedda,L. Negr1 and FGFR2 cooperatively regulate cortical development and core behaviors related to autism disorders in mice Brain 2018 Jul 27. doi: 10.1093/brain/awy190
Pischedda F, Cirnaru MD, Ponzoni L, Sandre M, Biosa A, Carrion MP, Marin O, Morari M, Pan L, Greggio E, Bandopadhyay R, Sala M and Piccoli G. LRRK2 G2019S kinase activity triggers neurotoxic NSF aggregation Brain. 2021 Apr 20:awab073. doi: 10.1093/brain/awab073.
Frapporti G, Colombo E, Ahmed H, Assoni G, Polito L, Randazzo P, Arosio D, Seneci P, Piccoli G. Squalene-Based Nano-Assemblies Improve the Pro-Autophagic Activity of Trehalose Pharmaceutics. 2022 Apr 14;14(4):862. doi: 10.3390/pharmaceutics14040862.
Marku A, Carrion MDP, Pischedda F, Marte A, Casiraghi Z, Marciani P, von Zweydorf F, Gloeckner CJ, Onofri F, Perego C, Piccoli G.The LRRK2 N-terminal domain influences vesicle trafficking: impact of the E193K variant. Sci Rep. 2020 Mar 2;10(1):3799. doi: 10.1038/s41598-020-60834-5.
