Overview
As the incidence of cancer continues to increase steadily among the world population, the available tools for understanding and treating cancer fail to meet our demand for cure. Powerful animal models emerge as a valid alternative; here, cancer cell biology, cancer evolution and treatment can be studied in an integrated way, leading to better understanding and precision therapies. The zebrafish model offers a number of advantages for experimental cancer biology, such as its rapid development, tractable genetics, suitability for in vivo imaging and for high throughput chemical screening. Cancer occurs naturally in zebrafish and can be induced with a variety of toxicological, environmental and genetic methods, making this small vertebrate an extraordinary tool in cancer research. Indeed, no other models offer the molecular toolbox, the superior imaging capabilities and the discovery power of genetic screens in just one tiny organism.
Our lab uses the zebrafish as a model for cancer biology. We have developed several zebrafish models of cancer that we use as a source of information on the biology of different cancer types in their endogenous environment. We can follow cancer predisposition, initiation, progression, response to therapies and relapse in the same model, a powerful platform that is well-suited for preclinical studies. We work in collaboration with basic/translational and clinical researchers to provide knowledge on the biology of different cancers that can be translated into therapeutic approaches for the immediate benefit of the patients.
Research directions
The projects we carry out in the lab are based on our models of cutaneous and ocular melanoma and pediatric glioblastoma.
- The role of extracellular vesicles in melanoma progression.
Extracellular vesicles (EVs) are membranous particles released by all cell types. Their role as functional carriers of bioactive molecules (e.g. RNA, DNA, lipids and proteins) is boosted in cancer. We have optimized a method for the isolation and characterization of zebrafish EVs from whole melanoma tissues. We found that melanoma EVs induce an inflammatory response through their non-coding (nc)RNA content, suggesting that they are a source of ncRNAs that can trigger inflammation in cells of the tumor microenvironment. This project aims at identifying the molecules and mechanisms of EV-induced tumor progression and tumor-associated inflammation.
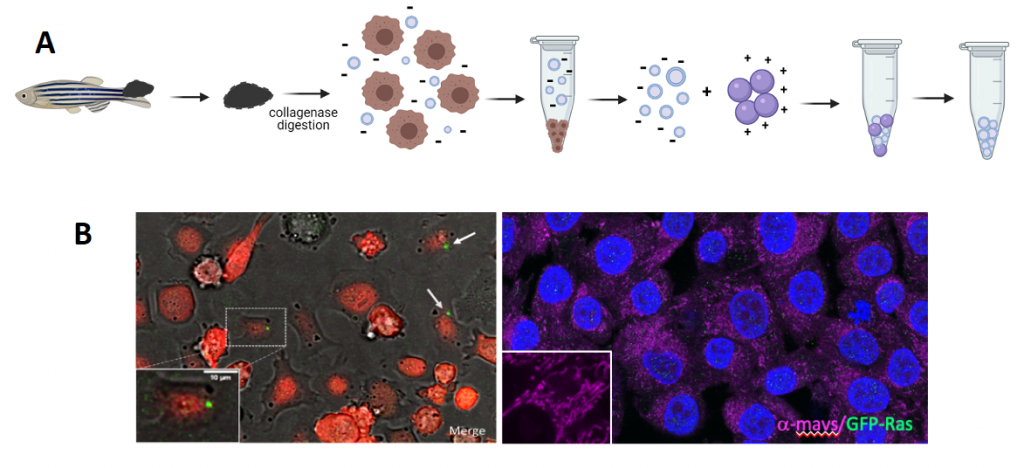
Figure 1: A. Schematic representation of melanoma microdissection, single cells dissociation, and iEVs isolation following the NBI method. B. Live image of coculture of Zmel cells with tEVs. B. Fixed A375 cells stained for GFP (zf tEVs) and mavs.
-
Alternative Telomere maintenance mechanisms trigger nucleic acid immune responses in glioblastoma
A large number of pediatric and 10% of adult brain tumors use Alternative Lengthening of Telomeres (ALT) to maintain telomere length and proliferation. A zebrafish model of pediatric brain cancer reproduces this mechanism (Idilli et al., 2020a, b) and shows increased cytoplasmic genomic DNA (C-Circles) and increased expression of Telomeric Repeat-containing RNA (TERRA). In this project, we want to investigate the potential of these nucleic acid species to activate the innate immune system, as a mean to induce an inflammatory response that worsen progression and survival in pediatric brain cancer patients. In collaboration with Cusanelli’s lab (link to Cell Biology and Molecular Genetics lab) we are investigating TERRA as a potential new therapeutic target to prevent ALT in brain tumors.
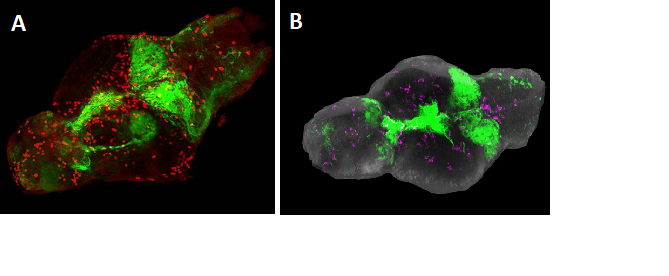
Figure 2: Brains from larval zebrafish expressing the GFP-Ras oncogene. A. Visualization of GFP-Ras fluorescence in green and immunostaining (red) for Phospho-histone 3 (PH3), indicating mitotic cells, in red. B. Microglia (magenta) in a zebrafish brain with tumor (green).
-
Uveal melanoma: models and metabolism
We are using the mitfa promoter to drive expression of the uveal melanoma oncogene GNA11Q209L in ocular melanocytes and have established a number of zebrafish lines that develop uveal melanoma earlier when associated with mutations in Bap1, Yap, APC or p53 genes. We are interested in understanding why melanocytes located in different anatomical positions throughout the body are susceptible to cancerous transformation by different oncogenic insults, despite a similar origin from neural crest. We reason that the local environment dictates changes that affect their responses to oncogenic events by rewiring their energetic metabolism. We are studying the metabolic rewiring of UM with different mutations to identify aspects of their metabolism that can be specifically targeted to halt tumor progression.
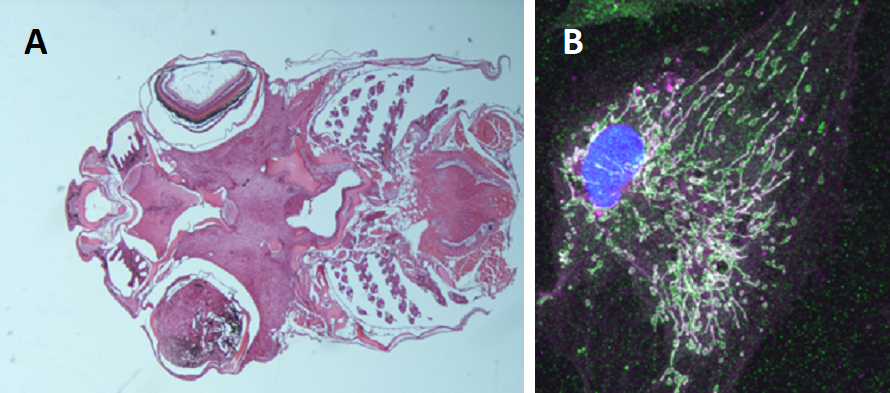
Figure 3: A. Uveal melanoma developing in a transgenic fish expressing human GNA11Q209L.B. Extensive mitochondrial network in a transformed melanocyte from a primary culture of a tumor with the same genotype.
Group Members
- Maria Caterina Mione, PI
- Francesca Lorenzini, Post-doc
- Federica Busi, PhD student
- Irene Pecchini, PhD student
- Vittorio Bontempi, PhD student
- Claudio Oss Pegorar, PhD student – in collaboration with “Cell Biology and Molecular Genetics” Lab (CIBIO, PI: Emilio Cusanelli)
- Antonella Calore, Pre-doc
- Alexia Grasso, visiting Post-doc
- Stephanie Marini, MSc student
- Giada Mazzurana, BSc student
Collaborations
- Maria L. Cayuela, Hospital Universitario, V. De La Arrixaca-IMIB-FFIS, Murcia, Spain
- Filippo del Bene, Sorbonne University, Paris, France
- Victor Mulero, University of Murcia, Spain
- Sergio Roman Roman (Institut Curie, Paris) and the UM Cure Consortium, France
- Eirini Trompouki, Institute for Research on Cancer and Aging Nice, University of Côte d' Azur, France
- Dirk Sieger, Centre for Neuroregeneration, The University of Edinburgh, U.K.
Selected Publications
Biagini V, Busi F, Anelli V, Kerschbamer E, Baghini M, Gurrieri E, Notarangelo M, Pesce I, van Niel G, D'Agostino VG, Mione M. Zebrafish Melanoma-Derived Interstitial EVs Are Carriers of ncRNAs That Induce Inflammation. Int J Mol Sci. 2022 May 14;23(10):5510. doi: 10.3390/ijms23105510.
Rosello M, Vougny J, Czarny F, Mione MC, Concordet JP, Albadri S, Del Bene F. Precise base editing for the in vivo study of developmental signaling and human pathologies in zebrafish. Elife. 2021 Feb 12;10:e65552. doi: 10.7554/eLife.65552.
Lefkopoulos S, Polyzou A, Derecka M, Bergo V, Clapes T, Cauchy P, Jerez- Longres C, Onishi-Seebacher M, Yin N, Martagon-Calderón NA, Potts KS, Klaeylé L, Liu F, Bowman TV, Jenuwein T, Mione MC, Trompouki E. Repetitive Elements Trigger RIG-I-like Receptor Signaling that Regulates the Emergence of Hematopoietic Stem and Progenitor Cells. Immunity. 2020 Nov 17;53(5):934-951.e9. doi: 10.1016/j.immuni.2020.10.007.
Idilli AI, Pagani F, Kerschbamer E, Berardinelli F, Bernabé M, Cayuela ML, Piazza S, Poliani PL, Cusanelli E, Mione MC*. Changes in the expression of pre-replicative complex genes in hTERT and ALT pediatric brain tumors. Cancers (Basel). 2020 Apr 22;12(4):1028. doi: 10.3390/cancers12041028.
Idilli AI, Cusanelli E, Pagani F, Berardinelli F, Bernabé M, Cayuela ML, Poliani PL, Mione MC*. Expression of tert prevents ALT in zebrafish brain tumors. Front Cell Dev Biol. 2020 Feb 11;8:65. doi: 10.3389/fcell.2020.00065.
Misselbeck K, Parolo S, Lorenzini F, Savoca V, Leonardelli L, Bora P, Morine MJ, Mione MC, Domenici E, Priami C. A network-based approach to identify deregulated pathways and drug effects in metabolic syndrome. Nat Commun. 2019 Nov18;10(1):5215. doi:10.1038/s41467-019-13208-z.
Gómez-Abenza E, Ibáñez-Molero S, García-Moreno D, Fuentes I, Zon LI, Mione MC, Cayuela ML, Gabellini C, Mulero V. Zebrafish modeling reveals that SPINT1regulates the aggressiveness of skin cutaneous melanoma and its crosstalk with tumor immune microenvironment. J Exp Clin Cancer Res. 2019 Sep 13;38(1):405. doi:10.1186/s13046-019-1389-3.
Precazzini F, Pancher M, Gatto P, Tushe A, Adami V, Anelli V, Mione MC*. Automated in vivo screen in zebrafish identifies Clotrimazole as targeting a metabolic vulnerability in a melanoma model. Dev Biol. 2019 Apr 15. pii: S0012-1606(18)30734-6. doi: 10.1016/j.ydbio.2019.04.005.
Zizioli D, Mione MC, Varinelli M, Malagola M, Bernardi S, Alghisi E, Borsani G, Finazzi D, Monti E, Presta M, Russo D. Zebrafish disease models in hematology: Highlights on biological and translational impact. Biochim Biophys Acta Mol Basis Dis. 2019 Mar 1;1865(3):620-633. doi: 10.1016/j.bbadis.2018.12.015.
Piragyte I, Clapes T, Polyzou A, Klein Geltink RI, Lefkopoulos S, Yin N, Cauchy P, Curtis JD, Klaeylé L, Langa X, Beckmann CCA, Wlodarski MW, Müller P, Van Essen D, Rambold A, Kapp FG, Mione MC, Buescher JM, Pearce EL, Polyzos A, Trompouki E. A metabolic interplay coordinated by HLX regulates myeloid differentiation and AML through partly overlapping pathways. Nat Commun. 2018 Aug 6;9(1):3090. doi: 10.1038/s41467-018-05311-4.
Chia K, Mazzolini J, Mione MC, Sieger D. Tumor initiating cells induce Cxcr4-mediated infiltration of pro-tumoral macrophages into the brain. Elife. 2018 Feb 21;7. pii: e31918. doi: 10.7554/eLife.31918.
Mayrhofer M, Gourain V, Reischl M, Affaticati P, Jenett A, Joly JS, Benelli M, Demichelis F, Poliani PL, Sieger D, Mione MC.* A novel brain tumour model in zebrafish reveals the role of YAP activation in MAPK- and PI3K-induced malignant growth. Dis Model Mech. 2017 Jan 1;10(1):15-28.
Anelli V, Mione MC*. Melanoma niche formation: it is all about melanosomes making CAFs. Pigment Cell Melanoma Res. 2016 Oct 23. doi: 10.1111/pcmr.12545.
You can find the full list of publications at this link.
