Overview
RNA-binding proteins (RBPs) and their impact on gene expression have long been studied in isolation. However, considering one RBP at a time leads to models of post-transcriptional regulation of gene expression that are unable to account for the sheer complexity of this regulatory layer. The main focus of the laboratory is thus to study the concurrent action of multiple RBPs by modeling post-transcriptional regulatory networks through computational and molecular biology tools. We are particularly interested in exploring the cooperative and competitive interplay of RNA-binding proteins, and how alterations of these mechanisms affect the onset and progression of cancer. 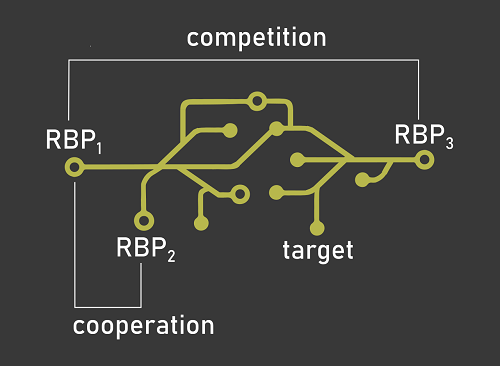
Research directions
-
Patterns of RBP interplay
Understanding if and how two RNA-binding proteins compete or cooperate will allow us to describe the effect of these interactions on their RNA targets and the related cellular processes. We design and implement algorithms to identify RBP interplay patterns by taking advantage of state of the art machine learning methods applied to transcriptome-wide protein-RNA interaction and expression datasets. -
Post-transcriptional networks in pancreatic adenocarcinoma
Pancreatic ductal adenocarcinoma is a lethal disease, with a 5% survival rate and rising incidence. The lack of effective cures calls for innovative treatment strategies. Post-transcriptional regulation of gene expression is broadly altered in this tumor: we analyze genetic alterations to RNA-binding proteins and study their consequences on post-transcriptional networks with the long-term goal to develop novel, interplay-based, therapeutic options for this disease. -
Epitranscriptomic networks in cancer
RNA modifications have emerged as a complex facet of post-transcriptional regulation. We analyze high-throughput RNA modification datasets and predict the cooperation and competition of the enzymes writing, reading, and erasing these marks, both within a modification and across different modification types. We use this knowledge to study how alterations to such mechanisms can affect the progression of cancer.
Group members
- Erik Dassi, PI
- Eliana Destefanis, PhD student
- Carlos Peula Cordero, PhD student
- Sébastien Bourdon, PhD student (co-mentored with Stefania Millevoi @ CRCT, Toulouse, France)
- Valentina Fambri, MSc student
Collaborations
- Stefania Millevoi, Cancer Research Centre of Toulouse, Toulouse, France
- Francisca Aguilo, Wallenberg Center for Molecular Medicine, Umeå University, Umeå, Sweden
- Lorenzo Montanaro, DIMES - University of Bologna, Bologna, Italy
- Alberto Inga, Department of Cellular, Computational and Integrative Biology - CIBIO, University of Trento, Trento, Italy
- Alessandro Quattrone, Department of Cellular, Computational and Integrative Biology - CIBIO, University of Trento, Trento, Italy
Funding
Bando: PRIN 2022 (D.D. 104/22)
snoRNA retaining transcripts (snoRTs) as post-transcriptional determinants of breast cancer cells phenotype
Erik Dassi, Responsabile di Unità
Codice Protocollo: 2022R8R3LB CUP: E53D23004920006

Selected publications
Herviou P, LeBras M, Dumas L, Hieblot C, Gilhodes J, Cioci G, Hugnot JP, Ameadan A, Guillonneau F, Dassi E.*, Cammas A*, Millevoi S*. (2020) hnRNP H/F drive RNA G-quadruplex-mediated translation linked to genomic instability and therapy resistance in glioblastoma. Nature Communications, doi: 10.1038/s41467-020-16168-x.
Rizzotto D, Zaccara S, Rossi A, Galbraith MD, Andrysik Z, Pandey A, Sullivan KD, Quattrone A, Espinosa JM, Dassi E.*, Inga A*. (2020) Nutlin-induced apoptosis is specified by a translation program regulated by PCBP2 and DHX30. Cell Reports. doi: 10.1016/j.celrep.2020.03.011.
Quattrone A, Dassi E. (2019) The architecture of the human RNA-binding protein regulatory network. iScience, 21:706-19. doi: 10.1016/j.isci.2019.10.058.
Dassi E. (2017) Handshakes and fights: the regulatory interplay of RNA-binding proteins. Front Mol Biosci, 4:67. doi: 10.3389/fmolb.2017.00067.
Editor Dassi E. (2016) Post-Transcriptional Gene Regulation: Methods and Protocols, Methods in Molecular Biology. Springer, doi: 10.1007/978-1-4939-3067-8.
Dassi E., Re A, Leo S, Tebaldi T, Pasini L, Peroni D, Quattrone A. (2014) AURA 2: Empowering discovery of post-transcriptional networks. Translation, 2(1): e27738. doi: 10.4161/trla.27738.
